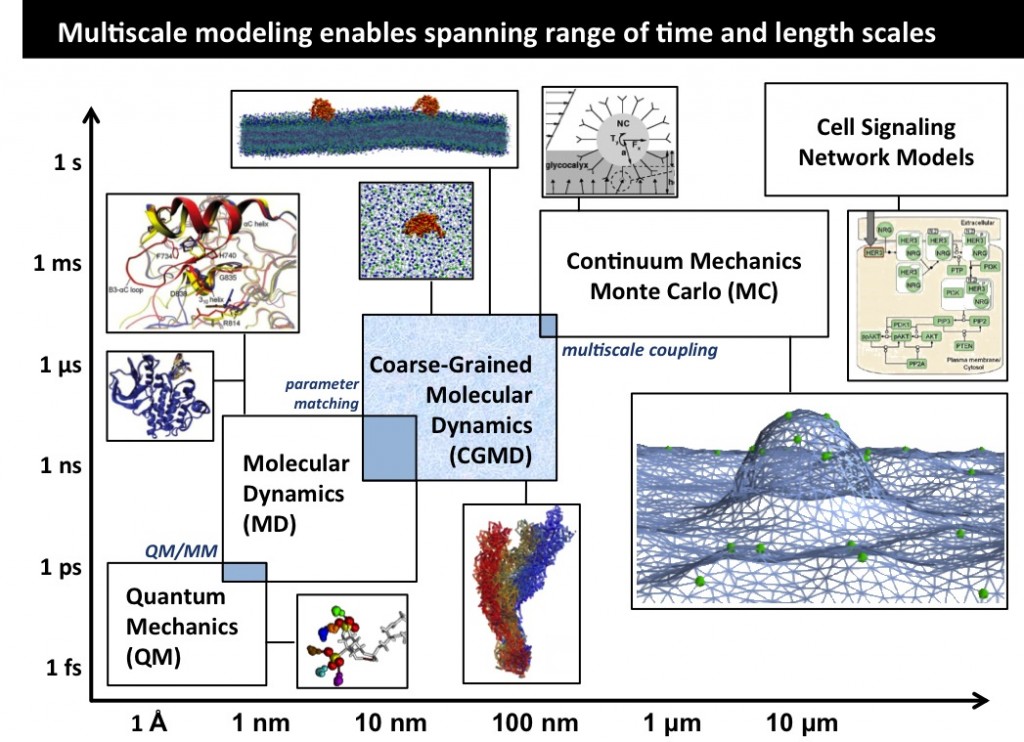
Multiscale Modeling
Our research interests lie at the interface of biomedical engineering and clinical pharmacology. Our goal is to provide integrative molecular, cellular, tissue, and organ level characterization of complex biomolecular systems and formulate quantitatively accurate microscopic models for predicting the interactions of various therapeutic agents with innate physiological systems. We employ several computational algorithms ranging from techniques to treat electronic structure, molecular dynamics, Monte Carlo simulations, stochastic kinetic equations, hydrodynamics, and complex systems analyses in conjunction with the theoretical formalisms of equilibrium and non-equilibrium statistical and quantum mechanics, and high performance computing in massively parallel architectures.
We incorporate features of nanocarrier design and optimization for clinical applications, including nanocarrier dimension, concentration, density of targeting molecules and characteristics of linkers used to attach targeting molecules into computational modeling that bridges the relevant multiple length and time scales. By developing and implementing computational methodologies, we will enable high-throughput simulations which will be experimentally validated. We will provide specialized computational techniques invoking the otherwise disparate molecular dynamics, statistical mechanics, hydrodynamics and particle interaction mechanics that define the landscape of multiple scales encompassing nanocarrier-based targeted drug delivery. This will yield a comprehensive model of nanocarrier dynamics that mechanistically handles the relevant transport and binding physical events which occur at multiple distinct scales. We will produce seminal algorithmic scale-bridging methodology for optimizing, via in silico calculation, nanocarrier design and delivery parameters as will be required clinically to provide safe and effective NC-based therapies. Proper choice of tunable physical characteristics otherwise is reachable only by conduct of huge numbers of experiments. Our work will facilitate fast-track disease treatment options by translation from nanocarrier design to medical practice. The investigators draw on synergistic expertise in engineering, biology and clinical medicine. One major aspect of our innovation is the simultaneous utilization and incorporation of seemingly disparate computational techniques required to manage the relevant computations of mechanistically definable events occurring as described at multiple scales. Another major aspect of our innovation is our collaborative use of synthesis capabilities to create prototype targeted nanocarrier for the validation experiments.
Next Generation Pharmacodynamic Models for Targeted Drug Delivery
Dynamics for Functionalized Nanocarrier Adhesion to Cell Surfaces in the Presence of Hydrodynamic Interactions: Vascular delivery of antibody-functionalized nanocarriers (50 nm-1 mm in size) to selectively bind targeted surface receptors expressed by endothelial cells is a viable therapeutic strategy in systems pharmacology. However, optimal nanocarrier design involves the appropriate selection of their size, shape, and antibody density on their surface. Additionally, the choice of appropriate tethers/linkers bridging the antibodies to the nanocarrier surface depends on the physiological microenvironment, including hemodynamics and rheological properties of blood flow in the microvasculature as well as the state of the endothelial cells (which governs expression of targeted receptors such as intracellular adhesion molecule-1, ICAM-1). We have recently shown that a model-based optimization of this design can be used to guide the achievement of targeting selectivity and specificity in vivo. We address the important aspects of nanocarrier adhesive dynamics in terms of how they impact binding selectivity and specificity. We employ technics ranging from direct numerical simulations for fluctuating hydrodynamics, brownian dynamics, and generalized Langevin dynamics.
[View Poster]
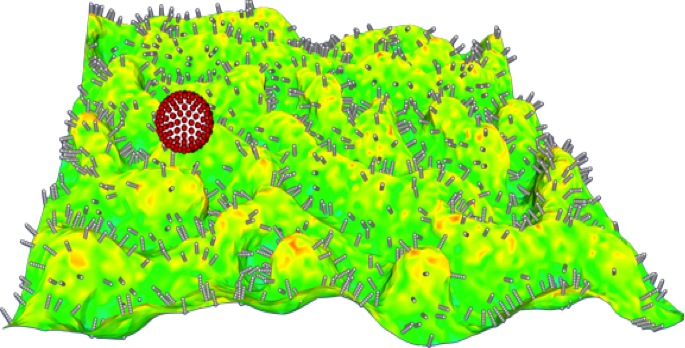
Physiologically Validated Models for Adhesion of Functionalized Nanocarriers to Endothelium in Targeted Drug Delivery: Targeting nanocarriers (NCs) loaded with drugs and probes to precise locations in the body may improve the treatment and detection of many diseases. Generally, to achieve targeting, the surface of the NC is coated with affinity ligands to enhance binding to molecules present on the cells of interest. Optimization of ligand density is a critical parameter in controlling NC binding to target cells. Other tunable properties include NC size and shape, surface chemistry (e.g., tethers of various lengths and stiffnesses) and deformability (such as gel-based NCs). Our objective is to develop a computational platform for modeling the targeting of functionalized NCs to optimize experimental design protocols for drug delivery. Recently we introduced a computational methodology based on Monte Carlo and the weighted histogram analysis method to calculate the absolute binding free energy between functionalized NC and endothelial cell surfaces. The calculated NC binding free energy landscapes have been validated against measurements performed in vivo as well as in cell-culture experiments. The effect of antibody surface coverage of NC on binding has been studied using the model that accounts for physiological factors such as hydrodynamic forces (e.g., shear stress) and resistance due to glycocalyx layers. We validated the simulations against three distinct classes of experiments: in vivo targeting in mice, targeting in cultured cells under flow, and binding assays using Atomic Force Microscopy. While higher ligand density always leads to higher binding avidity, it is not always the most effective. In a recent study, we investigated how NC avidity affects targeting to the pulmonary vasculature comparing NC binding to quiescent endothelium and inflamed endothelium. Expectedly, NC avidity was controlled by ligand density, with the higher avidity NCs demonstrating greater pulmonary uptake than lower avidity NCs in both naive and pathological mice. However, in comparison with high-avidity NCs, low-avidity NCs exhibited several-fold higher selectivity of targeting to pathological endothelium. This finding was translated into a Positron Emission Tomography imaging platform that was more effective in detecting pulmonary vascular inflammation using low-avidity NCs. Our computational modeling revealed that elevated expression of ICAM-1 on the endothelium is critical for multivalent anchoring of NCs with low avidity, while high-avidity NCs anchor effectively to both quiescent and activated endothelium. These results provide a new paradigm whereby computational model predictions are used to optimize NC targeting in pathological vasculature. In current work, we are developing multiscale computational strategies to incorporate NC adhesion to live cells, where membrane compliance and fluidity play dominant roles. In particular, we investigate the explicit effects of membrane stiffness and deformation on the NC adhesion landscape.
[View Poster]